Frequently Asked Questions About LumenPro
LumenPro Veterinary StrengthTM is a non-prescription eye drop formula recommended for use in pets with cataracts. It contains 0.375% lanosterol and 1% n-acetylcarnosine. LumenPro Veterinary StrengthTM is available only through veterinarians.
LumenPro veterinary strength offers a lower dose, higher concentration formula, .375%, 50% stronger than the consumer formulation at .25%. The stronger formulation improves pet owners’ compliance with treatment, requiring only 1 drop per day, at under $1/day.
LumenPro feedback from the vet community is favorable and clear. With limited ophthalmic specialists for referral and infrequent client follow-through, vets are excited to have a new treatment option and a veterinary-exclusive product.
The eye’s lens contains crystallin proteins, regularly aligned to allow light to pass through to the retina. Mammals naturally produce lanosterol, a molecule that prevents these aligned crystallin proteins from deforming and aggregating into cloudy cataracts that reduce clarity of vision. Reduced production of lanosterol often leads to the development cataracts.
Laboratory research has shown that a supplement of lanosterol to the eye can slow, halt and reverse the progression of cataracts. But since lanosterol is an insoluble molecule, it could only be delivered to the aqueous environment of the eye via injection. Our patent-pending process makes lanosterol soluble, so it can be delivered to the lens via an easy-to-administer topical solution.
N-acetylcarnosine (NAC) is an antioxidant. It scavenges for free radicals that cause age-related tissue damage. Unfortunately, NAC alone can take as long as nine months to produce noticeable results. We believe NAC is best used as a complement to the faster acting lanosterol. LumenPro Veterinary StrengthTM is the only commercially available eye drop that delivers both of these ingredients to the eye in a therapeutic dose.
The 2015 study, Lanosterol reverses protein aggregation in cataracts (Nature, 2015) demonstrated the effectiveness of lanosterol in treating cataracts in rabbits and dogs. A subsequent, seemingly contradictory paper, Failure of oxysterols such as lanosterol to restore lens clarity from cataracts, was conducted with human lens protein solubilization studies.
The premise that because all mammals have LSS lanosterol synthase pathways led to optimism that it would work on all mammals. While rabbits and dogs are on the clinical pathway to humans, lanosterol holds promise for human treatment, but has not been sufficiently studied.
We use aseptic processes, manufactured and packaged in a controlled environment per documented processes, ensuring the product and its packaging remain sterile from formulation to filling and packaging.
All manufacturing (vs terminal sterilization), steps are documented (due diligence). LumenPro is pursuing FDA approval and an NDC (national drug code)—while this applies primarily to human drugs, it is a requirement of some ecommerce channels, due to issues with human eye drop products.
Buyer beware. There are known issues with a number of consumer ecommerce veterinary medicines. The FDA took seven human eye drop products off the market 18 months ago, causing Amazon to drop all eye drops out of risk management. LumenPro has sought independent validation from a third-party lab for a number of eye drops claiming to contain lanosterol, and every competitive product tested to date has failed to meet its active ingredient claims.
Yes—LumenPro lanosterol drops have been tested for 24 months, through both accelerated and real-time stability tests.
Yes—LumenPro eye drops are suited for office-use and dispensing, in states that allow veterinarians to do so. Bulk discounts or drop-ship direct-to-client options are available.
Our guidance is a 90-day follow-up. New cataracts or insipient cataracts will see the quickest results. LumenPro 3-pack drops will ensure 90-day treatment in the consumer formulation.
Pet owners should watch for two changes: visible cloudiness, and behavioral change. Pet owners will, of course, struggle to diagnose ocular diseases in animals, but may notice changes like a new fear of stairs, or bumping into objects. They may notice behavioral improvement earlier than cloudiness.
The earlier the better. After the initial 90 days, many veterinarians recommend reducing treatment to a maintenance dose. (For example, reducing dosage from 2 drops 2x day to 1 drop 2x a day or 2 drops 1x day in the veterinary strength formula; consumer strength dosage is 2-3 drops 2x day). The maintenance dosage supplements the body’s natural lanosterol to the eye, if body isn’t producing enough lanosterol due to age, diabetes, etc.
LumenPro may also prove beneficial as a preventative medicine in animals whose age, breed, or condition like diabetes make cataracts extremely likely.
It is likely too late to reverse cataracts with lanosterol if the animal has already lost vision in the eye.
Now there are options. An ophthalmic referral may still be important to rule out a misdiagnosis. If you are able to determine that eye cloudiness is a cataract, and rule out nuclear sclerosis—cloudiness from the outside of the eye, not inside—consider lanosterol drops.
There are 2 contraindications—untreated diabetes and low tear production. LumenPro may still offer some benefits while changes are made to address diabetes. Schirmer’s tear test can help determine if the animal has lower than normal tear production.
If the animal experiences irritation or redness from LumenPro, discontinue and use saline or lubricant eye drop to clear it.
Prescribe enough drops to coat the surface of the eye. LumenPro has been used effectively on a wide range of animals, from dogs and rabbits, to horses and even a tiger. A recommendation of 1-4 drops is a reasonable guideline to help decide the right dose for your patient.
Schedule a follow-up in-office visit in 90 days to look for visible and/or behavioral change. If there is no change, the animal may be misdiagnosed or too advanced for a positive outcome, and a referral to an ophthalmic specialist is advised.
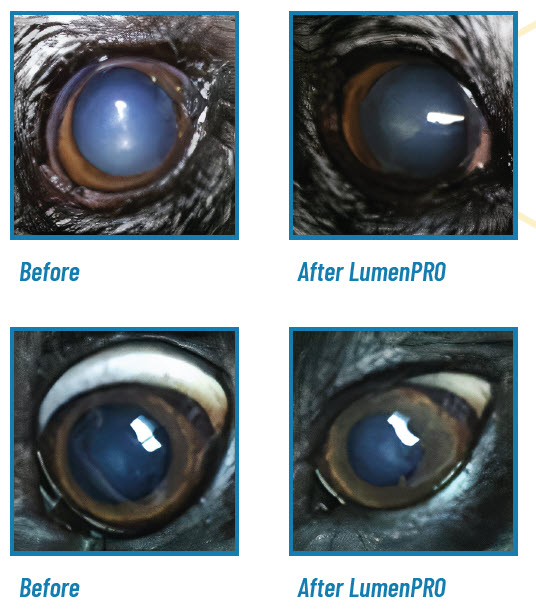
See the results for yourself
Become a Veterinary Partner
If you are a veterinarian, please sign up today to learn how LumenPro Vet Strength can benefit your patients, their owners, and your practice.
